Find the right manufacturing partner to bring the next frontier in MIS to life
Minimally invasive surgery or MIS is steadily gaining acceptance across the world today. With a CAGR of 9.8% from 2022 to 2030, MIS and surgical robots in particular is evolving to possess never before seen capabilities. Technological innovation has called for unprecedented levels of manufacturing expertise and production technology that is not always available with every medical device OEM (Original Equipment Manufacturer).
That’s why creators of next-gen minimally invasive surgical devices or surgical systems like Endoquest Robotics, have chosen Instrument Technology Inc. (ITI) – an experienced, equipped and reliable medical device contract manufacturer to partner with.
Why does experience matter?
A contract manufacturer who’s been in the business for more than a decade will be able to anticipate requirements and capabilities for the design and manufacture of medical device systems. Long time engineers who have worked in the production of medical devices and their components will have the experience to know what pitfalls exist, in order to prevent potential red flags further along in the life cycle of the medical device.
Why does equipment matter?
End to end manufacturing ensures that quality requirements are not only met but also exceeded. Strategies that can empower a contract manufacturer to provide best-in-class services to medical device OEMs are:
- Vertical integration
- Automation
- Miniaturization or manufacturing lines for miniature components
- Cleanroom facility
- Sterilization & packaging
Why should you choose a reliable contract manufacturer?
A reliable contract manufacturer is transparent in their working, with clear and consistent communication to keep you updated about the progress of your medical device’s design and production.
Medical device contract manufacturers who have offshore production facilities may not be able to provide a clear line of communication, or have a stable supply chain and logistics, as one who has an onshore production facility.
A manufacturing partner that has cutting edge capability
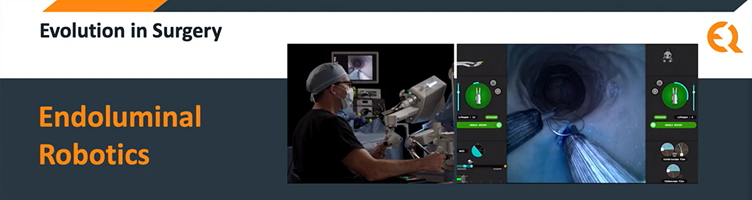
Capability to handle the next frontier in minimally invasive surgery: “Endoluminal Surgery” – it’s not the final frontier, but certainly the latest in the voyages of minimally invasive surgical device manufacturers. Surgeons have progressed from open surgeries, to laparoscopic surgery to robotic surgery. The later requiring a small incision for the catheter to enter the body.
The difference: Endoluminal surgery utilizes the body’s natural orifices to access the surgical sites with high precision. Precision that’s so high, that you will be empowered to collect a tissue sample from with an organ’s lumen and then surgically suture the patch – thus enabling faster healing and minimum discomfort to patients.
It’s every surgeon’s dream to be able to perform a surgery that causes the least discomfort, less time to perform and is easier to control. Endoquest Robotics is a pioneer in the field of Endoluminal surgery -designing and manufacturing the entire surgical robotics setup. This robotic surgical system is now ready to run tests at distinguished centres like the Harvard Medical school, Mayo Clinic, Cleveland Clinic and Advent Health.
Instrument Technology Inc., (ITI) is proud to partner with Endoquest, supporting them with our 50+ years of product manufacturing engineering experience and expertise; State-of-art equipment in our 24,000 sq. foot, ISO 13485:2016, ISO 9001:2015 certified, FDA-approved facility; and our track record of being a reliable manufacturing partner.
Learn more about this fascinating innovation: the world’s first flexible endoluminal robotic surgery system here.
Contact us today to learn more about our services.
.